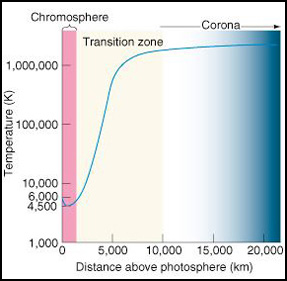
The Calcium Filtered Sun
Although the Sun is completely made of gas, the density and temperature of the gas changes drastically as you travel from the outermost regions to the center. Scientists look at different layers of the Sun by using telescopes with different filters.
Our Sun emits radiation in almost every wavelength. In addition to the visible light we can detect with our eyes, the Sun also emits radio waves, infrared and ultraviolet radiation as well as x-rays and gamma rays. Lucky for us we can focus in on a specific layer of the Sun by using a filter to remove all but one type of radiation.
The Calcium K (Ca-K) image allows us to see some of the magnetic structure on the Sun. The bright sections show where the magnetic field is the strongest.
Calcium K (Ca-K) telescopes and filters are used to study the wavelength of 393.4 nm. This emission line is one of two that are produced by Calcium just at the edge of the visible spectrum, in a layer that is slightly lower and cooler than the layer viewed in Hydrogen-alpha. The emission line displays areas of super granulation cells that are brightest and strongest in areas of high magnetic fields, such as sunspot activity and active regions. Having the ability to study the Calcium K and the Hydrogen-alpha line provides important insights into the structure, strength, and depth of these active regions.
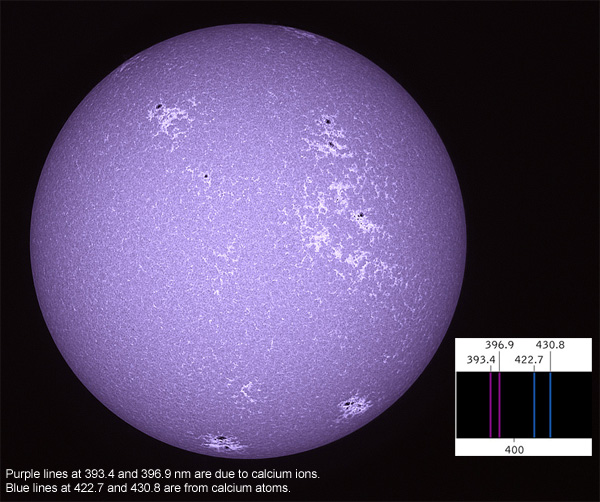
The dark lines (missing parts of the spectrum) occur because the Sun is composed of many elements including hydrogen, helium, calcium, iron, sodium and magnesium to name but a few. Due to the high temperature and pressure, these elements are in the form of a hot ionised gas. These elements absorb energy at a very specific wavelength of light unique to them. This occurs when an electron jumps from an orbit with low energy to an orbit with a higher energy; the energy is absorbed from the spectrum and this produces the dark (absorption) line.
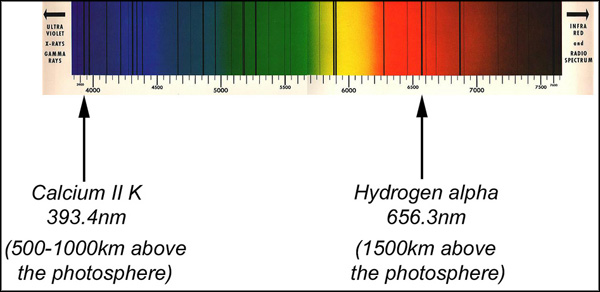
Above the photosphere is the chromosphere at around the height of 2-3,000km and the corona 10-20,000km, both are transparent to visible light; however the spectrum in these regions are dominated by emission lines. This is when the ionised gases reach a certain temperature and pressure where an electron from a higher energy state moves to a lower energy state and emits a photon. In the chromosphere, Hydrogen alpha emits this photon at a wavelength of 656.3nm, and Calcium II K (393.3nm) and H (396.8nm).
These electron shifts occur at a very specific temperature and pressure which is unique to each element, in turn this is directly linked to the height above the photosphere in which these conditions are right. We observe these emissions from a height determined by the opacity. As Calcium II K and H and Hydrogen alpha create different degrees of opacity, we see their emissions (and the structures revealed by their illumination) at different heights in the chromosphere.
The chromosphere is by no means a homogenous body of gas either. Spicules are huge columns of plasma rising and falling every 15 minutes and are present up to a height of 9,000km; these are drawing energy upwards and contributing to the heating of the chromosphere and corona. The temperature increases in height above the photosphere.